
Junior Principal Investigator
Cancer Immunotherapy, Stress Respone, Biomarker
xulongyong(at)szbl.ac.cn
2025-Present The Shenzhen Bay laboratory Institute of Cancer Research Junior Principal Investigator
2024-2025 MD Anderson Cancer Center Senior Research Scientist
2021-2024 Baylor College of Medicine Instructor
2015-2021 Baylor College of Medicine Postdoc
2013-2015 Shanghai Institute of Biochemistry and Cell Biology,CAS Associate Researcher
2007-2013 Shanghai Institute of Biochemistry and Cell Biology,CAS PhD.
2003-2007 Huazhong Agricultural University Bachelor
Despite revolutionizing cancer treatment and conferring long-term benefits,including curesto a subset of patients, immunotherapy fails to elicit a response in approximately 70% of cases. Our laboratory is addressing this critical challenge through collaborative networks with leading hospitals and research institutions. Utilizing extensive clinical tumor samples and a diverse array of preclinical mouse models, our research is focused on two primary aims: 1) To systematically elucidate the adaptive mechanisms underlying tumor immune escape under therapeutic stress conditions such as chemotherapy, with the goal of defining the molecular pathways that suppress immunogenicity and leveraging this knowledge to develop innovative strategies for improving response rates; and 2) To develop novel biomarkers that enable accurate, rapid, and dynamic monitoring of patient responses to immunotherapy.
Dr. Xu has achieved several research breakthroughs in the fields of tumor therapy resistance, immune microenvironment, and stem cell aging by focusing on the endoplasmic reticulum stress (ER stress) response and the ER-associated degradation (ERAD) pathway.Heisthe first to discover the molecular mechanisms by which the ER stress pathway mediates chemotherapy resistance and immune evasion in tumors (Cell, 2024;J Clin Invest.,2018). Based on this discovery,theycollaborated with Fosun Pharma to conduct the first-in-human clinical trial of the small molecule inhibitor ORIN1001 (NCT03950570).ThisPhase 1 clinical evaluation of ORIN1001 has demonstrated tolerabilityandclinical responses (SD and PR) in multiple patients with heavily-pretreated advanced solid tumors.
His research on the ERAD pathway has elucidated the mechanisms through which hematopoietic stem cells and T cells maintain proteostasis within the immune microenvironment (Nat. Cell Biol., 2020;eLife, 2021). These findings have provided novel insights into the regulatory mechanisms of stem cell aging and have identified potential targets for innovative therapeutic strategies to combat hematopoietic stem cell exhaustion.
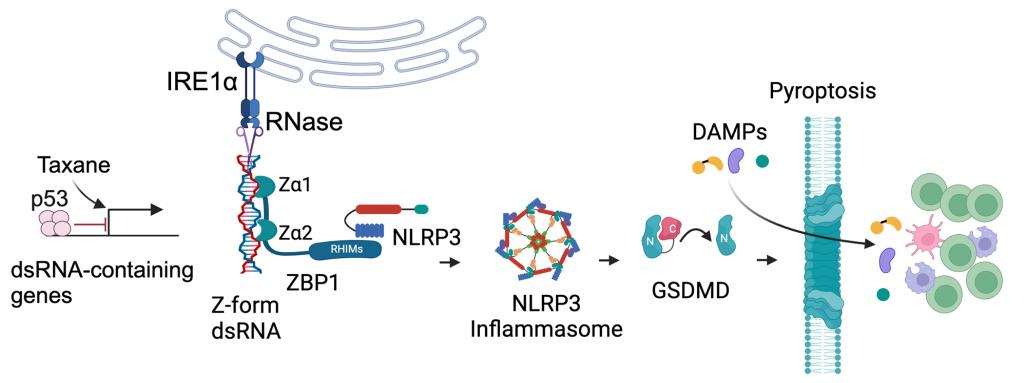
The ER stress sensor IRE1αmediates immune evasion in triple-negative breast cancer (TNBC).
The ancient ER stress sensorIRE1a RNase silences taxane-inducedimmunogenic z-typedouble-stranded RNA (dsRNA)through regulated IRE1-dependent decay (RIDD) to prevent NLRP3 inflammasome-dependent pyroptosis.Inhibition of IRE1aby ORIN1001inTrp53-/-TNBC allows taxane to induce extensive dsRNAs that are sensed by ZBP1,which in turn activates NLRP3-GSDMD-mediated pyroptosis. Consequently, IRE1a RNase inhibitor plus taxaneconverts PD-L1-negative, ICI-unresponsive TNBC tumors into PD-L1high immunogenic tumors that arehyper-sensitive to ICI.
1.Xu L,Peng F, Luo Q, Ding Y, Yuan F, Zheng L, He W, Zhang SS, Fu X, Liu J, Mutlu AS, Wang S, Nehring RB, Li X, Tang Q, Li C, Lv X, Dobrolecki LE, Zhang W, Han D, Zhao N, Jaehnig E, Wang J, Wu W, Graham DA, Li Y, Chen R, Peng W, Chen Y, Catic A, Zhang Z, Zhang B, Mustoe AM, Koong AC, Miles G, Lewis MT, Wang MC, Rosenberg SM, O’Malley BW, Westbrook TF, Xu H, Zhang X, Osborne CK, Li JB, Ellis MJ, Rimawi MF, Rosen JM, Chen X. “IRE1α silences dsRNA to prevent taxane-induced pyroptosis in triple-negative breast cancer”.Cell, 2024;187, 7248-7266.
·Research highlight: Bordon, Y. Targeting ER stress sensor restores immunogenicity of chemotherapy.Nat Rev Immunol, 2024;24, 848.
·Research highlight: Robinson N, Polara R, Thomas D. “IRE1α: a gatekeeper of chemotherapy-induced immunogenicity in triple-negative breast cancer”.STTT, 2025; 10:52.
2.Xu L,Liu X, Peng F, Zhang W, Zheng L, Ding Y, Gu T, Lv K, Wang J, Ortinau L, Hu T, Shi X, Shi G, Shang G, Sun S, Iwawaki T, Ji Y, Li W, Rosen J, Zhang X, Park D, Adoro S, Catic A, Tong W, Qi L, Nakada D, Chen X. “Protein quality control through endoplasmic reticulum-associated degradation maintains haematopoietic stem cell identity and niche interactions”.Nat Cell Biol, 2020;22, 1162-1169.
·Research highlight: Lam, K., Signer, R.A.J. ERADicating stem cells from their niche.Nat Cell Biol22, 1155–1157 (2020).
3.Zhao N#, Cao J#,Xu L#, Tang Q, Dobrolecki LE, Lv X, Talukdar M, Lu Y, Wang X, Hu DZ, Shi Q, Xiang Y, Wang Y, Liu X, Bu W, Jiang Y, Li M, Gong Y, Sun Z, Ying H, Yuan B, Lin X, Feng XH, Hartig SM, Li F, Shen H, Chen Y, Han L, Zeng Q, Patterson JB, Kaipparettu BA, Putluri N, Sicheri F, Rosen JM, Lewis MT, Chen X. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer.J Clin Invest.,2018;128, 1283-1299. (#:co-first author).
4.Liu X#, Yu J#,Xu L#, Umphred-Wilson K, Peng F, Ding Y, Barton B.M, Lv X, Zhao M.Y, Sun S, Hong Y, Qi L, Adoro S. and Chen X. “Notch-induced endoplasmic reticulum-associated degradation governs mouse thymocyte β−selection”.eLife, 2021;10, e69975. (#:co-first author).
5.Zheng L#,Xu L#, Xu Q, Yu L, Zhao D, Chen P, Wang W, Wang Y, Han G, Chen CD. “Utxloss causes myeloid transformation”.Leukemia, 2018; 32, 1458-1465. (#:co-first author).
6.Xu L, Zhang W, Zhang X and Chen X. “Endoplasmic Reticulum Stress in Bone Metastases”.Front. Oncol., 2020;10, 1100.